The Interlaboratory Comparison (ILC) CS-cfDNA_2025 is the first step in our 3-Phase ILC scheme. Tailored specifically for laboratories working in oncology, molecular pathology, liquid biopsy, and related fields, this ILC offers a unique opportunity to evaluate the performance of your methods for quantifying (C) and size-profiling (S) of cfDNA and ctDNA prior to dPCR/ddPCR and NGS.
Why should I participate?
By participating, you will become part of a growing network dedicated to method standardization and will be listed as a co-author in a scientific publication on the standardization of circulating tumor DNA (ctDNA) measurements using well-characterized reference materials (RMs).
What are the samples?
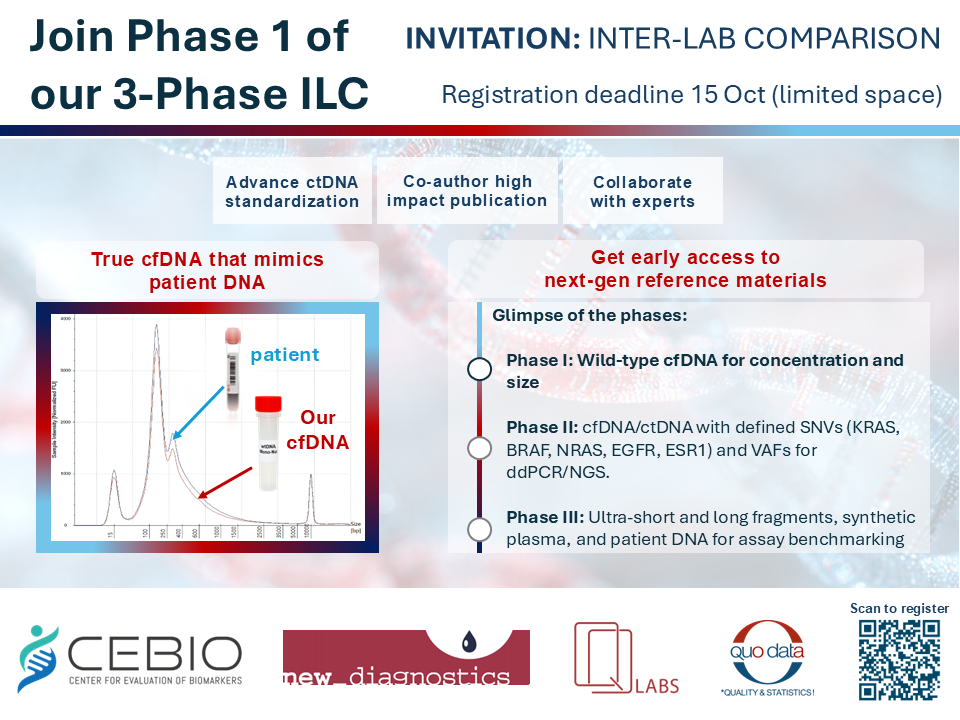
Each participant will receive two vials of highly characterized wild-type mono-nucleosomal cfDNA derived from HMEC-1 cells. This RM is produced through natural degradation, not by artificial methods such as mechanical shearing or enzymatic digestion. As a result, it closely resembles native patient cfDNA and represents a new, optimized standard available on the market.
Is this Interlab study accredited?
QuoData is an 17043 accredited PT provider. Despite this scheme not being implemented in the current scope, we apply all requirements from ISO/IEC 17043:2023. Homogeneity & Stability testing is performed according to ISO 13528:2022-08.
What is measured?
- cfDNA Concentration – Directly measured from the provided buffer
- cfDNA Fragment Size Profile – Evaluated to confirm integrity and distribution
What is the price for participation?
59 € per sample bundle for each laboratory
What is the timeline?
Registration is possible until October 15th. Sample shipment is planned for late Fall 2025*. Analyzes of the samples and submission of results is possible until the end of 2025.
How are the results submitted?
Results are submitted anonymously via QuoData’s online PT portal.
What will we get at the end?
Participants will receive a certificate of participation and a detailed performance report. Additionally, with participant agreement, results may contribute to a peer-reviewed scientific publication. EQA participants will also benefit from a 20% discount on other products in our portfolio, priority access to early testing of new products, and first consideration for custom RM production requests.
Will this ILC only happen once?
No, this is the first ILC in our 3-Phase ILC scheme. We are actively working to provide a range of circulating tumor DNA (ctDNA) mutations at varying variant allele frequencies (VAFs) to support internal quality control and method standardization. Additionally, we aim to offer the option of using our well-characterized wild-type RM, allowing you to perform in-house VAF dilutions.
Please check liquid-biopsy.new-diagnostics.com
* We reserve the right to postpone or to cancel the ILC if the required minimum number of participants is not reached.